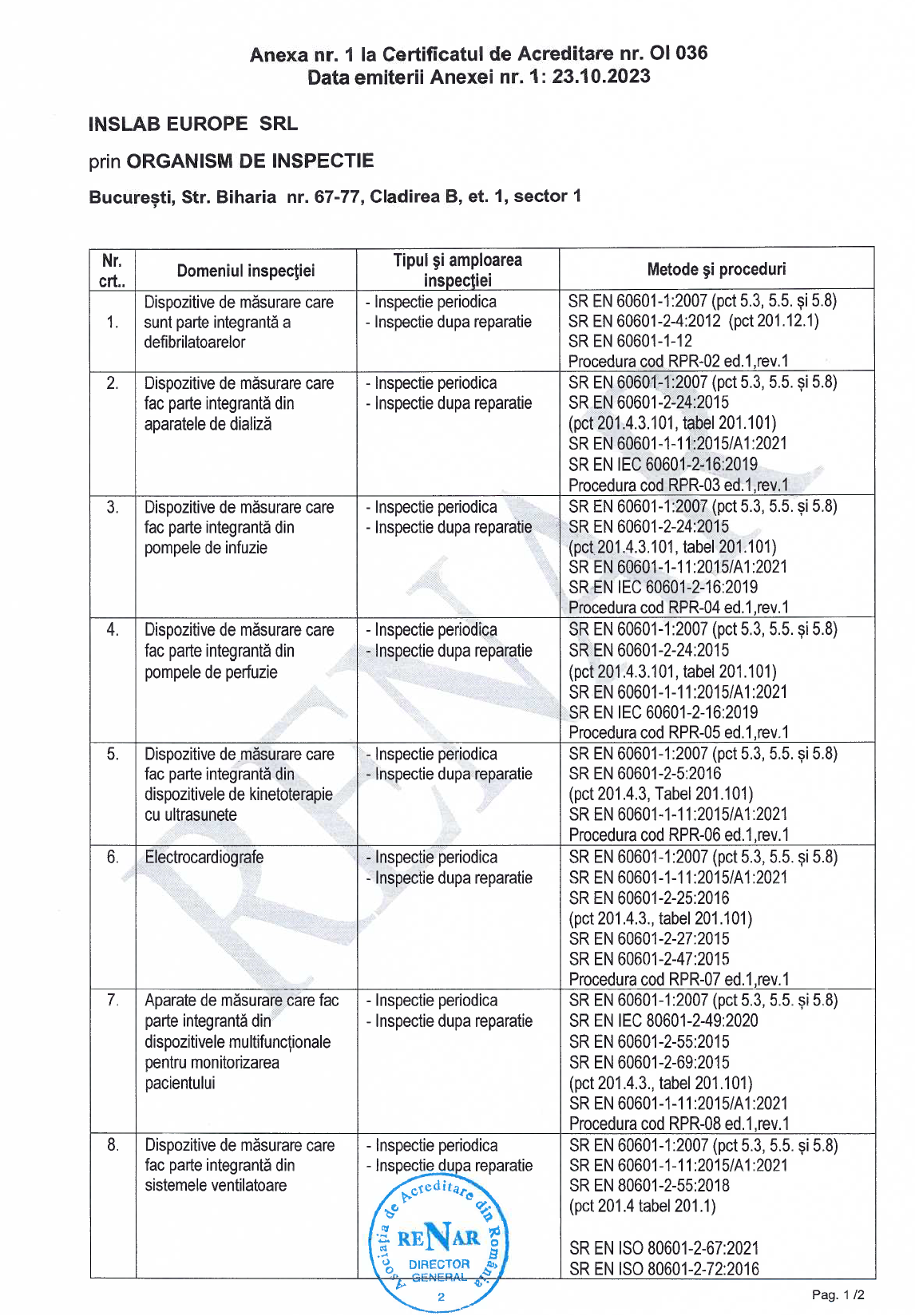
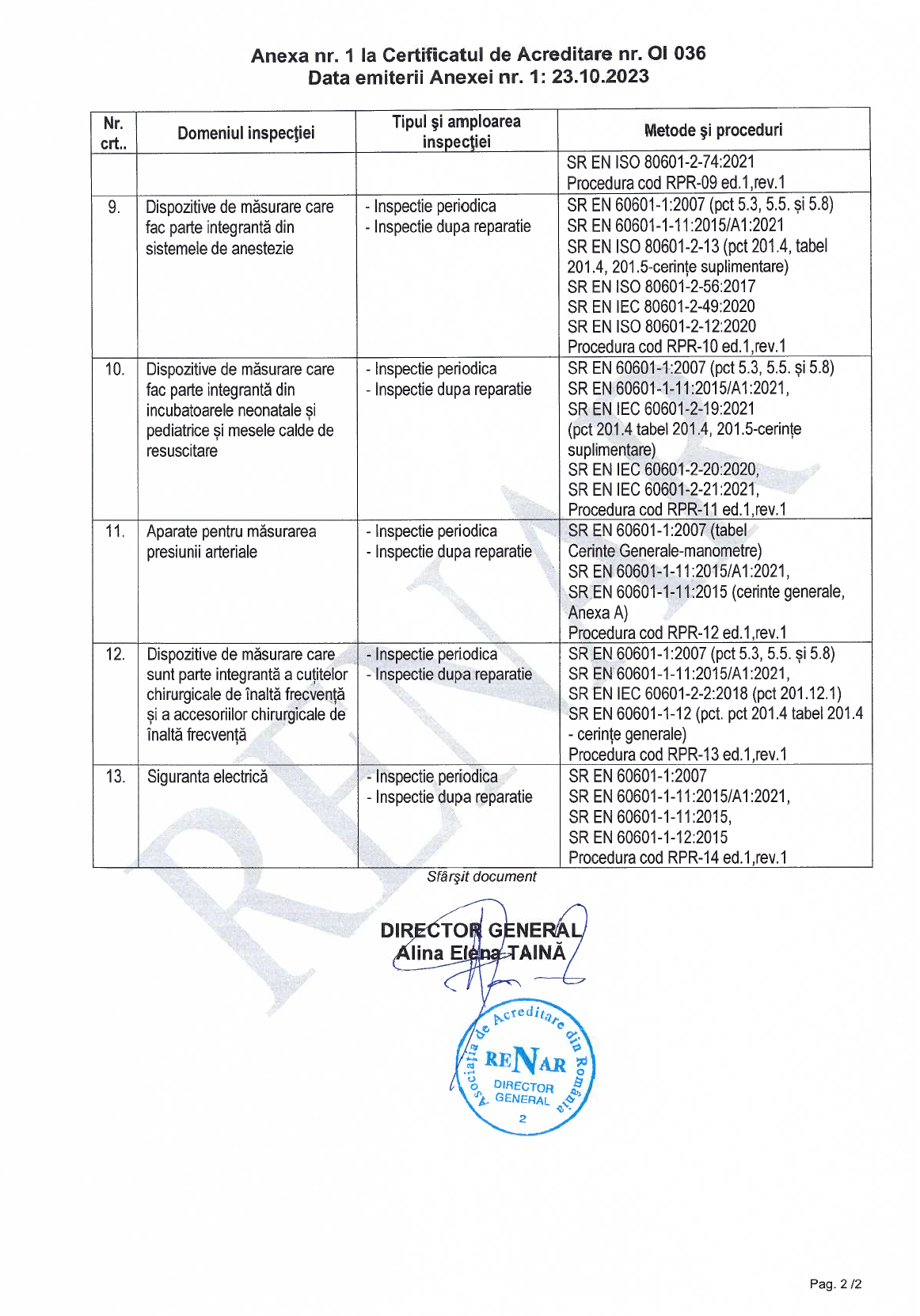
Why choose Meteuropescan verification services
To consistently deliver top-notch services, we systematically identify essential processes and allocate the necessary resources to manage our business with reliability and success. We meticulously document and control all pertinent activities, ensuring thorough planning and provision of resources, as well as clear assignment of responsibilities and authorities. The services offered by Meteuropescan Romania are committed to always meeting the satisfaction of our clients and complying with relevant authorities. In cases where expectations are not met, a comprehensive review and improvement process is initiated.